Background:
Relapsed/refractory acute myeloid leukemia (r/r AML) has a poor prognosis, and most patients fail standard chemotherapy and hematopoietic stem cell transplantation or relapse after treatment. We previously reported and evaluated CLL-1-targeted chimeric antigen receptor T-cell therapy in adult r/r AML patients, demonstrating for the first time the efficacy and tolerable safety of CLL-1 CAR-T cell therapy in adult r/r AML patients. On this basis, we expanded the data volume and continued to evaluate the safety and efficacy of CLL-1 CAR-T in AML with dose escalation.
Methods:
This is a single-center, phase I, dose-escalation trial of CLL-1 CAR-T cell in adult patients with r/r AML (ChiCTR2000041054)admitted to Tianjin First Central Hospital from January 2021 to June 2023. All patients received a lymphodepleting chemotherapy with cyclophosphamide (500mg/m2) and fludarabine (30mg/m2) 3 days before CAR-T cell infusion, followed by 4 incremental doses of CAR-T cells: dose level (DL)1 (0.5×10^6/kg),DL2(1×10^6/kg),DL3(1.5×10^6/kg)and DL4(2×10^6/kg)CAR-T cells. The primary objective of the trial was to evaluate safety and tolerability, and to determine the recommended Phase 2 dose. Key secondary objectives included the assessment of overall response rate, complete response rates as well as progression free survival and overall survival.
Results:
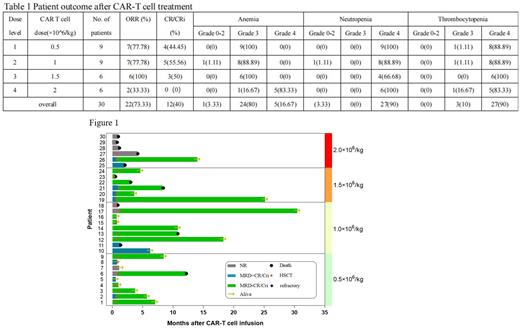
Thirty patients were enrolled and treated in the dose-escalation phase of the study. The median age of the patients was 38 years (range 18-73), and the median number of prior lines of treatment was 4(2-10), 5(16.67%) patients progressed from MDS to AML, 13(43.33%) patients relapsed after treatment, of which 8(26.67%) patients had previously received HSCT. There were 9, 9, 6 and 6 patients receiving DL1, DL2, DL3 and DL4, respectively. All patients experienced cytokine release syndrome (CRS) (100%): grade 0-2(n=18), grade 3(n=11), grade 4(n=1), and only one patient (3.33%) experienced grade 4 neurotoxicity (NTX), which improved after plasma exchange. In addition, all patients experienced hematological toxicity, with 29 patients (96.67%) developing grade 3/4 granulocytopenia, 28 patients (93.33%) developing grade 3/4 anemia, and 30 patients (100%) developing thrombocytopenia. Patients who received haploidentical bridge transplantation after infusion had normal granulocyte, erythrocyte, and platelet transplantation and did not develop serious infection, suggesting that when severe agranulocytosis occurs after CAR-T infusion, bridge transplantation can reverse this toxicity (Table 1).
Efficacy evaluation showed that 73% of patients responded to the infusion, with 12(40%) patients achieving MRD-CR/CRi and 10(33.33%) patients achieving MRD+CR/CRi, for an overall response rate (ORR) of 73.33% (Figure 1). All patients who underwent bridging haploidentical HSCT after CAR-T cell infusion achieved MRD-CR/CRi, and the median time to transplantation was 0.7 months (0.3-1.13). The median OS rate of patients after transplantation was higher than that of patients without transplantation after infusion (P=0.015), as was PFS (p=0.044). The maximum tolerated dose was not determined as no dose-limiting toxic effects were observed. Responses were observed at all dose levels, with a median follow-up of 132 days (range 15-914 days). Patients had a median PFS of 300 days (95% CI, 191.320 to 408.680) and a median OS of 348 days (95% CI, 234.011 to 461.989).
The expansion of CLL-1CAR-T cells was tracked by flow cytometry, and the median peak of CAR-T expansion occurred at day 11 (range 2-15) after infusion. When comparing the peak CAR-T expression in CR/CRi and NR patients, the proportion of peak CAR-T cells was significantly higher in CR/CRi patients.
Conclusion:
CLL-1 is a potential therapeutic target in AML. The proportion of patients who developed severe CRS was low and only 1 patient experienced NTX. Although severe agranulocytosis may occur, patients receiving CLL-1CAR-T therapy may have the opportunity to achieve CR/CRi prior to transplantation, which may reduce the risk of relapse and mortality, and prolong patient survival with encouraging results. Due to the small sample size, no relevant dose-response relationship was observed, and the trial is currently enrolling more cases and will be progressively expanded to Phase II clinical trials.
Disclosures
No relevant conflicts of interest to declare.